Married male with children, who was asked to write on three different subjects concerning women’s healthcare by the ConsumerSafety.Org . Although I have worked in the healthcare product industry, I am not a doctor. All three of the healthcare issues I discuss scream for solutions as to what has been done, what should have been done, and how they impact women. I have no doubt if these problems impacted men as much as they do women, a Congress made up mostly of men would have addressed each issue far sooner. Clinical Trials Fact: Women make up over half of the U.S. population. As reported by the GAO, women have been underrepresented in NIH supported clinical research which lead to unidentified differences in treatment results between men and women when
Topics:
run75441 considers the following as important: Consumer Safety, Featured Stories, Healthcare, Hot Topics, Maternal Mortality, politics
This could be interesting, too:
Robert Skidelsky writes Lord Skidelsky to ask His Majesty’s Government what is their policy with regard to the Ukraine war following the new policy of the government of the United States of America.
NewDealdemocrat writes JOLTS revisions from Yesterday’s Report
Joel Eissenberg writes No Invading Allies Act
Ken Melvin writes A Developed Taste
Married male with children, who was asked to write on three different subjects concerning women’s healthcare by the ConsumerSafety.Org . Although I have worked in the healthcare product industry, I am not a doctor.
All three of the healthcare issues I discuss scream for solutions as to what has been done, what should have been done, and how they impact women. I have no doubt if these problems impacted men as much as they do women, a Congress made up mostly of men would have addressed each issue far sooner.
Clinical Trials
Fact: Women make up over half of the U.S. population.
As reported by the GAO, women have been underrepresented in NIH supported clinical research which lead to unidentified differences in treatment results between men and women when incurring a disease. There have been instances of women experiencing different and also adverse effects to medications and treatments than what was experienced by men in NIH Clinical trials. It is thought the NIH’s Inclusion Policy established requirements governing women’s inclusion in its clinical research may have led to this issue.
Some study examples:
The Baltimore Longitudinal Study of Aging started in 1958 did not include women until 1978 in its study even though women lived 6 years longer than men. 1000 men were initially in the study and no women. Another study, the Physicians Health Study concluded in 1989, the taking of low-dose aspirin might lower your risk for heart disease. It included 22,000 men and zero women. A study investigating the possible interactions between the libido-boosting drug Flibanserin (also known as “female Viagra”) and alcohol used a study group of 25 participants which included twenty-three men. It raises the question, why and how would we ever know the impact of drugs on women if only men are used in trials?
Why the Under Representation?
The driving factor for the lack of women in tests is not necessarily driven by bias as much as a lack of knowledge of the biological differences determining how disease symptoms may present in each gender. A broad-based assumption was made of the test findings of men. The results could also apply to women? The testing of men is simpler as men are not subject to the hormonal fluctuations of women. As sound(?) as this reasoning may be in minimizing the number of trials, this appears to be more of a financial decision.
Reports of birth defects from the use of Thalidomide during the 1950s and 60s lead to FDA guidelines excluding potential child bearing women from participation in Phase 1 and 2 clinical studies until reproductive toxicity studies were conducted and evidence of effectiveness and safety was available. The FDA guide lines were misinterpreted and applied to all clinical study phases even though it was not intended to exclude women.
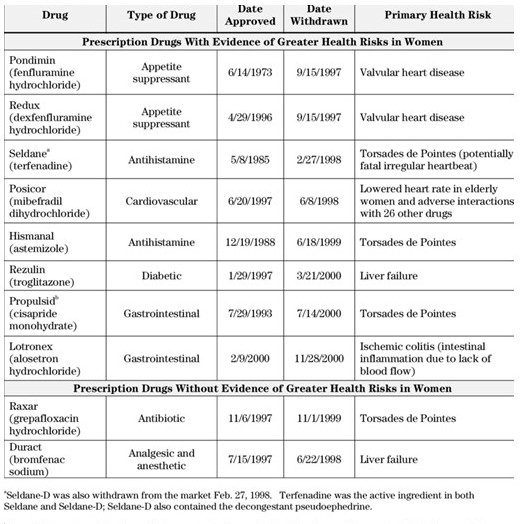 Dangers of Under Representation
Dangers of Under Representation
Assumptions from an 11 year NIH study on moral development in children using only boys concluded little girls are morally inferior to little boys. Females are simply different and arrive at conclusions different than men and just as moral. Eight of 10 approved drugs were pulled from the market due to health risks for women which were not risky for men. More women than men used four of the drugs and for 4 other drugs women and men used them equally. The differences in men and women between the two sets revealed itself in the later set of 4 with women experiencing serious side effects more often than men. Hence, emphasizing the need to have women equally represented in clinical studies. “Excluding women makes a difference: If women had been included before 1978, the link between osteoporosis-calcium-estrogen and progesterone would have been discovered in time to help their mothers”.
Resolution
In 1993 the Health Revitalization Act was passed which incorporated the use of women and minorities in NIH clinical studies. In 2000, Congress asked the GAO to assess the progress of the NIH. While progress was made to include women and minorities in trials, the GAO recommended the NIH to improve reporting format. Later years the GAO again assessed the NIH recommending the NIH improve the consistency of reporting by sex so as to allow researchers to “identify potentially important sex differences that may ultimately affect patient care.”
Globally the representation in 43% women and 57% men in clinical trial representation. In the US, the representation has improved to 49% women and 51% men. There is still work to be done. Most recently, the 21st Century Cure Act was passed with one of its intents being to move new drugs to market faster through testing in the public sector. “Without detailed clinical trials and studies, there is effectively no way to determine the extent of potential side effects and other issues the current detailed trials and studies provide.” Numbers predicting probability versus clinical trial experience, we will have to see how this plays out.
Essure
In November 2016, the FDA issued a “boxed warning” for the permanent female sterilization device Essure device after reports of it causing perforation, abdominal pain, and serious complications. A “boxed warning” is a type of warning appearing on the package insert for certain prescription drugs or devices. The Food and Drug Administration specifies the warning be formatted with a box or border around the text and is done when there is reasonable evidence of a serious hazard when used. It is the strongest warning the FDA requires.
Essure is a permanent female sterilization device consisting of metal coils which eventually embed into a woman’s fallopian tubes creating scar tissue blocking sperm access to a woman’s eggs. It is reversible only through surgery. In February 2016, the FDA designated Essure to have a “boxed warning” which is meant to alert doctors as to hazards of the device.
In February 2018, a group of women calling themselves the E-Sisters met with former FDA Commissioner Scott Gottlieb. The E-sisters believe Essure has caused themselves and tens of thousands of others health problems, from bleeding, bloating, and pelvic pain to more obscure symptoms such as rashes, tooth loss, joint pain and fatigue associated with an allergic or autoimmune reaction. They brought with them a photo album of other E-Sisters who had suffered because of Essure and also Madris Tomes, a former FDA analyst. Ms. Tomes’s software company tracks adverse medical events reported to the FDA and had logged 26,000 events caused by Essure.
Asking whether a ban might be possible, Commissioner Gottlieb confirmed anything is possible. On March 7, 2018, Gottlieb confirmed Essure would remain on the market. In its history, the FDA has only banned two products.
The original manufacturer Conceptus put Essure through a Level III approval process and presented its data to a FDA advisory committee “touting its near-perfect effectiveness in preventing pregnancy and its high levels of satisfaction among women.” Later studies challenged the initial studies and effectiveness. A Yale study challenged the rigor of the Level III process. a JAMA study reported 5% of all women using these devices required follow up surgery, and a third study claimed women using Essure were 10 times more likely to require surgery.
After November 2017, the U.S. was the only country in the world where Essure was still available after new owners Bayer removed Essure from every other market for “commercial reasons” and not because of safety. Bayer announced in July 2018, it would also remove the device from the US market after December 31st, 2018 due to declining sales.
Maternal Mortality
Healthcare for women Maternal mortality is an important indicator of a nation’s overall quality of healthcare.
Even though maternal mortality worldwide dropped 44% between 1990 and 2015 830 women ; die every day from causes related to pregnancy and while giving birth of which much is preventable. 99% of all those maternal deaths occur in developing countries. WHO has launched an initiative to meet the needs of women in developing countries by addressing access to and the quality of reproductive, maternal, and newborn healthcare services. Everyone would agree the effort is necessary in developing countries.
One would think the maternal rate of death in a highly developed nation such as the US would be lower when compared to other and similar nations. Why not? With the advent of the PPACA, many preventative healthcare measures were put in place for women and Medicaid was expanded in many states. US citizens spend far more for healthcare and have greater or similar access to healthcare. And yet every year in the U.S, 700 to 900 women die from pregnancy, or birth-related causes, and an approximate 65,000 almost die due to complications. Contrary to what healthcare should be, the US ranks low in providing maternal healthcare in the developed world.
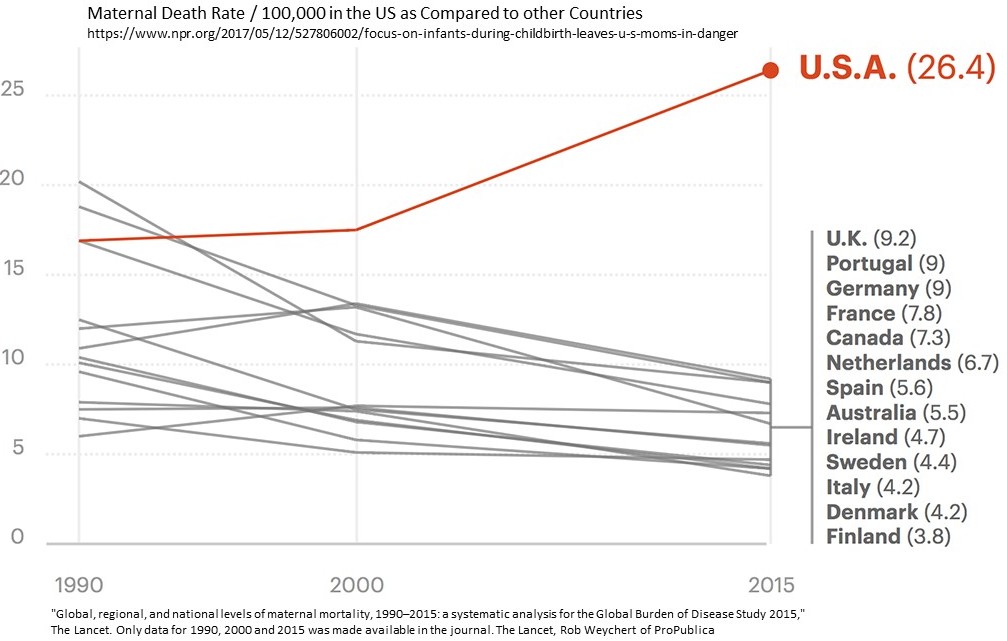 Even with the PPACA, expanded Medicaid in place; and when compared to their Canadian sisters, American women are three times more likely to die from the start of a pregnancy up till one year after the birth of a child (defined by the Centers for Disease Control). The death rate for American women is 26.4 deaths per 100,000 as opposed to 7.3 deaths per 100,000 in Canada (Chart). The ratio worsens when compared to Scandinavia countries as American women are six times as likely to die as Scandinavian women.
Even with the PPACA, expanded Medicaid in place; and when compared to their Canadian sisters, American women are three times more likely to die from the start of a pregnancy up till one year after the birth of a child (defined by the Centers for Disease Control). The death rate for American women is 26.4 deaths per 100,000 as opposed to 7.3 deaths per 100,000 in Canada (Chart). The ratio worsens when compared to Scandinavia countries as American women are six times as likely to die as Scandinavian women.
There are two stories, one for economically secure women and another for minority, native American, rural, and lower income women. The statistics worsens for women of color with their being more likely to die in pregnancy or childbirth and are nearly four times more likely to die from pregnancy-related causes than white women. In high-risk pregnancies, African-American women are 5.6 times more likely to die than white women. Amongst women diagnosed with pregnancy-induced hypertension (eclampsia and pre-eclampsia), African-American and Latina women were 9.9 and 7.9 times in danger of dying than white women with the same complications. Native American and Alaskan Native women experience similar discriminatory care. Half of all U.S. births are covered by Medicaid and covers women up to two months past delivery leaving a substantial gap after child birth when other issues can arise.
Barbara Levy, vice president for health policy/advocacy at the American Congress of Obstetricians and Gynecologists; “We worry a lot about vulnerable little babies and we don’t pay enough attention to those things catastrophic for women.”
The emphasis has been on safe baby care and safe birthing which lead to a significant decline in baby mortality. As reported in a Propublica, NPR report, the difference in “maternal mortality numbers contrast sharply with the impressive progress in saving babies’ lives.” Maternal death rates while giving birth and up to one year later has increased by an approximate 10 deaths per 100,000 since 2000 till 2015 or greater than the 9.2 deaths per 100,000 in the U.K, (Chart).
The problems occur before, during, and after delivery.
Mary D’Alton, chair of ob/gyn at Columbia University Medical Center and author of papers on disparities in care for mothers and infants. “The training was quite variable across the U.S., there were some fellows that could finish their maternal-fetal medicine training without ever being in a labor and delivery unit. When I had my own child I realized, ‘Oh my goodness. That was completely insufficient information.'”
And doctors fail to heed the warning signs women are alerting them too.
Elizabeth Howell, professor of obstetrics and gynecology at the Icahn School of Medicine, Mount Sinai Hospital; “The way that we’ve been trained, we do not give women enough information for them to manage their health postpartum. The focus had always been on babies and not on mothers.”
With 39 weeks of a good pregnancy, the expectant mother went to the hospital to induce labor. Inducing typically ends up with a cesarean delivery. 23 hours later, the mother delivered normally a healthy baby girl with the only occurrence being sharp pains in the kidney area alleviated with more epidural. In 20 hours, a healthy mother before the birth of her daughter died.
The pain came back 90 minutes after the birth. Upon her doctor husband questioning the ob/gyn, he was told it was acid reflux, and a common reaction after birthing. The pain increased, her blood pressure spiked at 169/108, and her husband asked the OB whether this could be preeclampsia.
Her blood pressure upon admission was 147/99, she experienced similar readings during labor, and for one period of 8 hours no readings were made. All eyes were on the health of the baby, not on the mother, and what could be coming to pass. For a woman with normal blood pressure such as this young mother, a blood pressure reading of 140/90 would be indicative of preeclampsia.
Her husband reached out to another doctor, he anxiously relayed the symptoms, and she quickly diagnosed what the young mother was suffering from . . . a disorder called HELLP syndrome or Hemolysis, (a breakdown of red blood cells); Elevated Liver enzymes; and Low Platelet count. A disorder if not treated quickly leads to death.
This is only a brief recital of the tragedy which befell Lauren Bloomstein and her husband Larry. With additional delays in finding a surgeon, Lauren began to experience bleeding in her brain which would lead to paralysis. She knew she was dying before her husband’s eyes. A neurosurgeon was called in to relieve the pressure and stop the bleeding. Since her platelets were low he could not operate, the hospital did not have an adequate supply, and by the time additional supply arrived it was too late. She was brain dead and was allowed to pass on after her daughter was placed next to her one last time.
The warning signs of life-endangering problems were there and were missed (pain in the kidney area) or ignored (abnormal high blood pressure for Lauren), excuses for pain (reflux) were made, and pain killers administered to dull the pain and other symptoms (blood pressure) not explored while she deteriorated in front of her husband who suspected preeclampsia. The missing part of this was the protocol to diagnose early on and prevent Lauren from slipping into late stages of preeclampsia. This is not an isolated incidence as the deaths of women giving birth keep increasing as evidenced in the attached chart.
This is but one story as told by NPR and Propublica. There are many more stories of tragedy which go untold.
