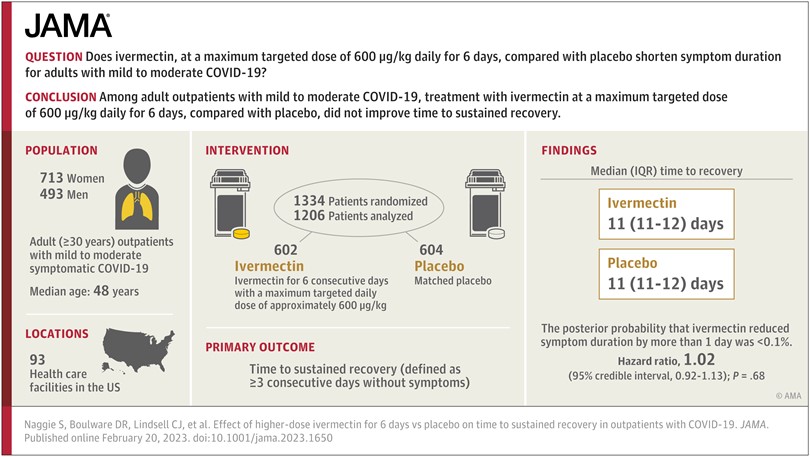
Will a high dose of Ivermectin for 6 Days impact Covid? As a biochemist, I find the argument of a lack of mechanism for Ivermectin as a justification for prescribing it for COVID particularly pernicious. While it is correct to say that we didn’t know the mechanism for aspirin for decades after we knew it worked (one could say the same about the smoking and lung cancer), that’s not a logical basis for prescribing a drug. The gold standard is a double-blind, randomized, placebo-controlled trial. Such was reported, February 20, 2023. “Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial, Clinical Pharmacy, and Pharmacology.” JAMA | JAMA Network,
Topics:
Angry Bear considers the following as important: Education, Healthcare, politics
This could be interesting, too:
Robert Skidelsky writes Lord Skidelsky to ask His Majesty’s Government what is their policy with regard to the Ukraine war following the new policy of the government of the United States of America.
Joel Eissenberg writes No Invading Allies Act
Ken Melvin writes A Developed Taste
Bill Haskell writes The North American Automobile Industry Waits for Trump and the Gov. to Act
Will a high dose of Ivermectin for 6 Days impact Covid?
As a biochemist, I find the argument of a lack of mechanism for Ivermectin as a justification for prescribing it for COVID particularly pernicious. While it is correct to say that we didn’t know the mechanism for aspirin for decades after we knew it worked (one could say the same about the smoking and lung cancer), that’s not a logical basis for prescribing a drug.
The gold standard is a double-blind, randomized, placebo-controlled trial. Such was reported, February 20, 2023.
“Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial, Clinical Pharmacy, and Pharmacology.”
JAMA | JAMA Network, Susanna Naggie, MD, MHS1,2; David R. Boulware, MD, MPH3; Christopher J. Lindsell, PhD4.
Question: Does ivermectin, with a maximum targeted dose of 600 μg/kg daily for 6 days, compared with placebo, shorten symptom duration among adult (≥30 years) outpatients with symptomatic mild to moderate COVID-19?
Findings: In this double-blind, randomized, placebo-controlled platform trial including 1206 US adults with COVID-19 during February 2022 to July 2022, the median time to sustained recovery was 11 days in the ivermectin group and 11 days in the placebo group. In this largely vaccinated (84%) population, the posterior probability that ivermectin reduced symptom duration by more than 1 day was less than 0.1%.
Meaning: These findings do not support the use of ivermectin among outpatients with COVID-19.