I have written on women being included in clinical trials in 2019. The article I was asked to write included Clinical Trials, reviewing the issues with Essure, and also Maternal Mortality. Three issues which had much detail and information, difficult to cover it all , and could have been much longer. A Woman’s Right to Safe Healthcare Outcomes, Angry Bear. This article is more recent and includes additional and more up to date detail. The conclusion being (at least to me) there is still issues with Clinical Trials including women. What I have made into an article in a learning module. Including Women in Clinical Research | Health Inequities, Whitman-Walker Institute’s LGBTQ+, HIV Care and Prevention Training, Eleanor Sarkodie, August 2023
Topics:
Bill Haskell considers the following as important: Clinal TRials, Healthcare, Hot Topics, women
This could be interesting, too:
NewDealdemocrat writes JOLTS revisions from Yesterday’s Report
Joel Eissenberg writes No Invading Allies Act
Bill Haskell writes Families Struggle Paying for Child Care While Working
NewDealdemocrat writes January JOLTS report: monthly increases, but significant downward revisions to 2024
I have written on women being included in clinical trials in 2019. The article I was asked to write included Clinical Trials, reviewing the issues with Essure, and also Maternal Mortality. Three issues which had much detail and information, difficult to cover it all , and could have been much longer. A Woman’s Right to Safe Healthcare Outcomes, Angry Bear.
This article is more recent and includes additional and more up to date detail. The conclusion being (at least to me) there is still issues with Clinical Trials including women. What I have made into an article in a learning module.
Including Women in Clinical Research | Health Inequities, Whitman-Walker Institute’s LGBTQ+, HIV Care and Prevention Training, Eleanor Sarkodie, August 2023
The historical roots of this underrepresentation can be linked back to, among other factors, a general considerations document published by the Federal Drug Administration (FDA) in 1977.
- The consequences of thalidomide use in pregnant women spurred new guidance on excluding women of child-bearing potential from phase 1 and phase 2 research trials. Researchers often assumed women (in this context cisgender women) would have the same response to drugs as men, and viewed them as more complex and expensive participants due to fluctuating hormone levels and reproductive potential.
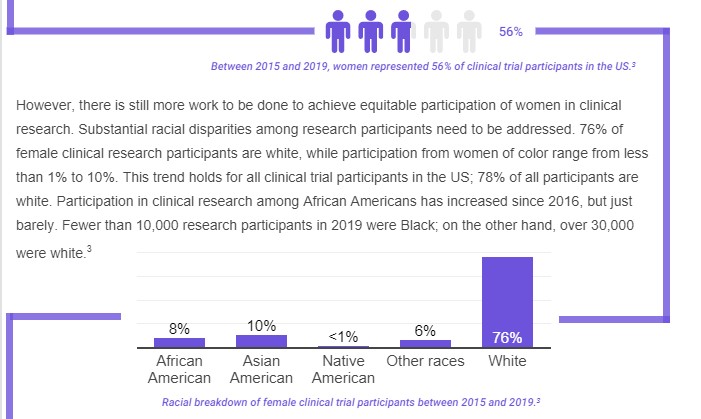
- The impact of these short-sighted guidelines resulted in decades-long underrepresentation of women in clinical trials research. A study published in 2019 investigated the magnitude of female underrepresentation in clinical studies performed or published between 1966 and 2018. It found that while women made up 49% of all participants enrolled during that time, they were substantially underrepresented in the majority of disease categories examined.
Underrepresentation hindered progress on understanding women’s responses to medications for decades. In 1990, the National Institutes of Health founded the Office of Research on Women’s Health with a mandate to increase women’s participation in research. Finally in 1993, the FDA reversed the 1977 guidance with another guidance that lifted the ban of women of child-bearing potential in early phase research. The decision to include women is now left to researchers, regulatory bodies, and women themselves.
Even so, female underrepresentation in clinical research persisted after the 1993 guidance until 2016. Since early 2016, women represent the majority of participants in clinical trials; male participation has been on the decline since 2017. Women now represent the majority of clinical trial participants in the US, there is still a lack of data distinguishing between cisgender women and women of the transgender experience.
Disparities in clinical trials participation.
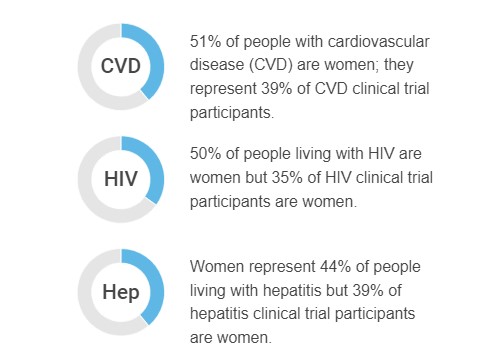
Women are especially underrepresented in clinical trials focused on cardiovascular disease, HIV, and hepatitis.
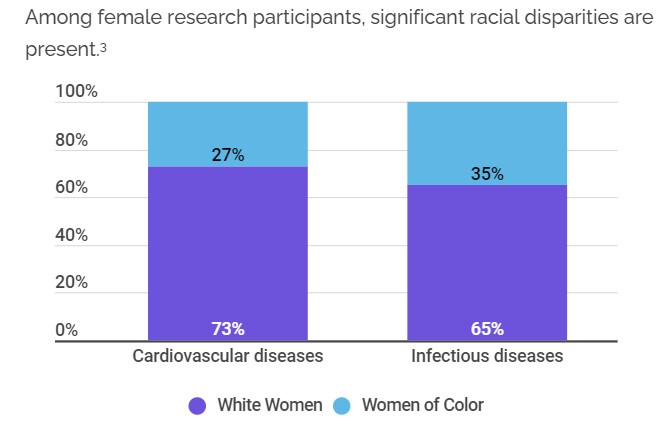
Racial Factors in clinical trials
Not much has changed with including women in medical trials since I wrote on it.